Research
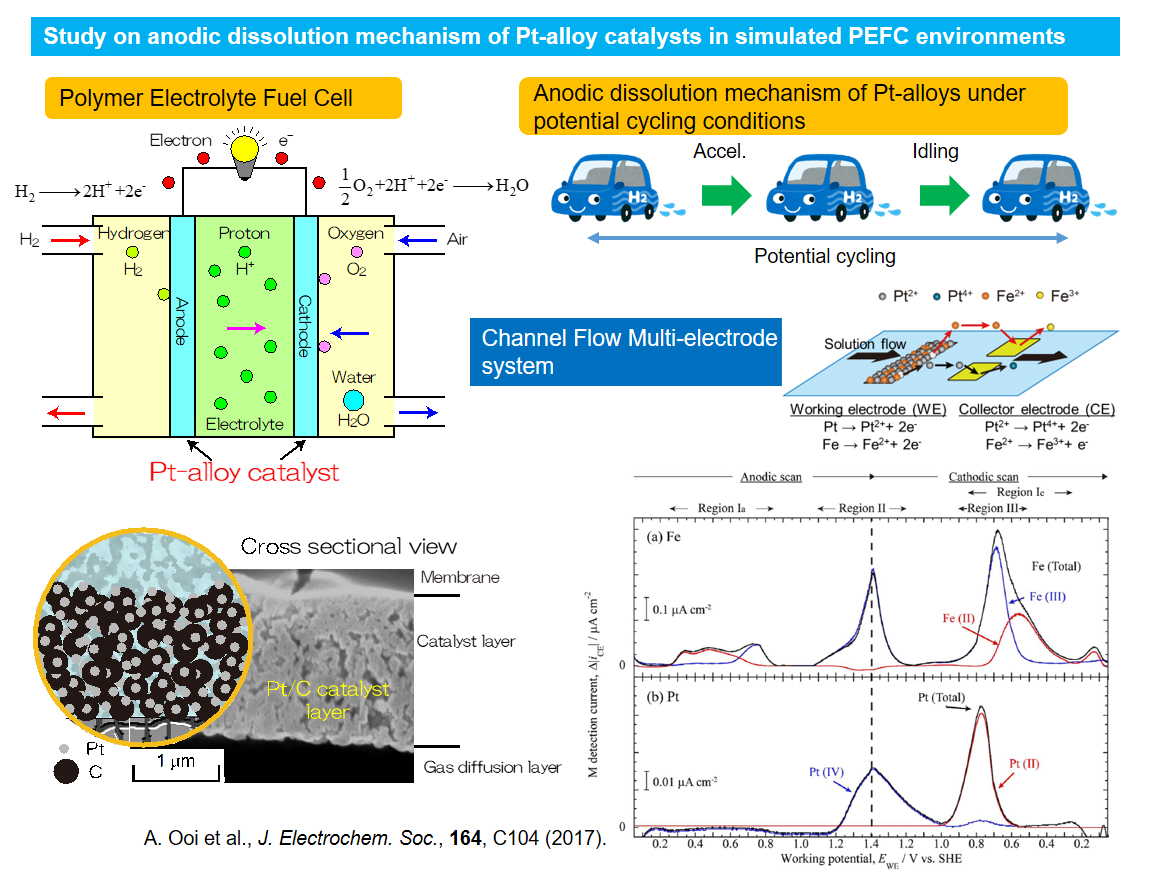
Study on anodic dissolution mechanism of Pt-alloy catalysts in simulated PEFC environments
The polymer electrolyte fuel cell (PEFC) is one of a power generating chemical devices utilizing oxygen and hydrogen as fuels. PEFC has many advantages such as no production of hazardous materials, working at relatively low temperature. Therefore, PEFC is expected as an alternate energy conversion and efficient power source device. However, one of the key factors limiting the use of PEFC is durability and reactivity of Pt catalysts especially at a cathode. We are investigating degradation mechanisms of Pt and Pt-alloy catalysts in PEFC environments by using channel-flow multi-electrode system, a unique electrochemical method enabling to detect dissolved metallic ions.
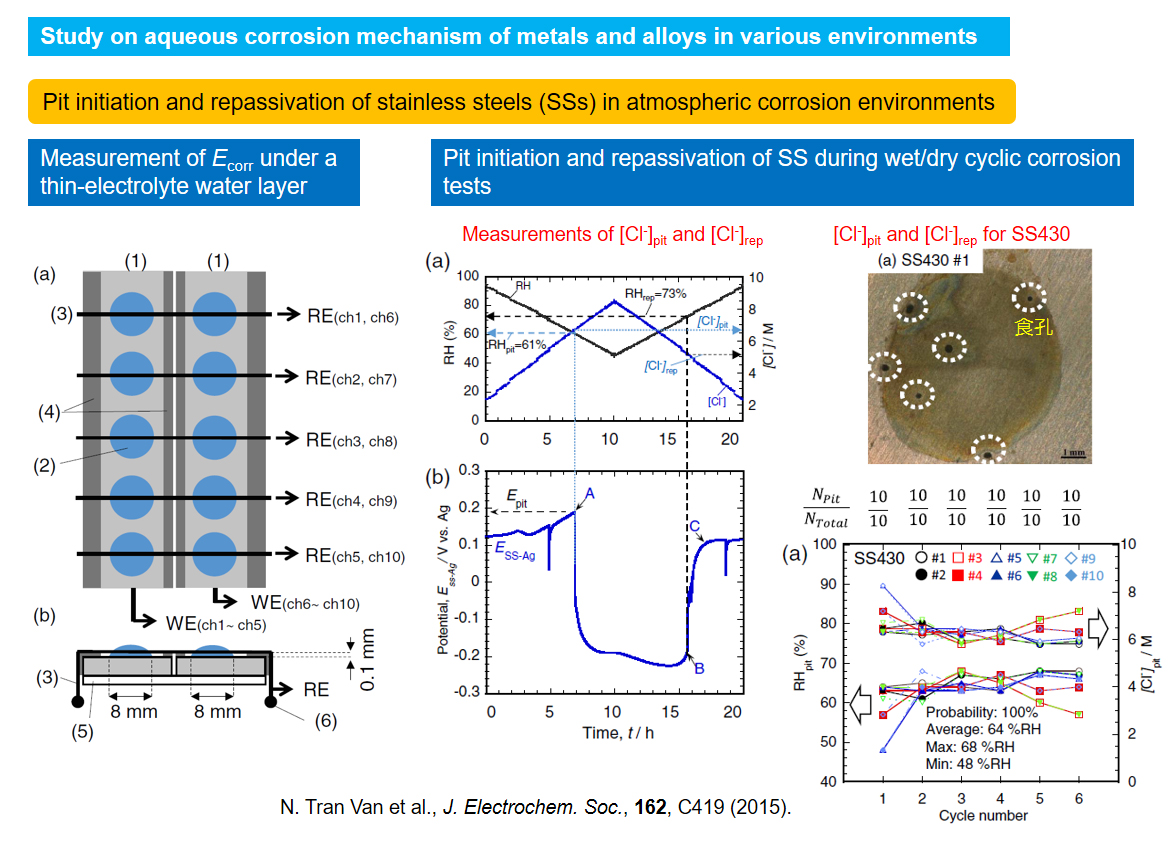
Study on aqueous corrosion mechanism of metals and alloys in various environments
Most of metallic materials suffers from corrosion or environmental degradation in usage environments. Aqueous corrosion of metallic materials are electrochemical reactions consisting of anodic dissolution of metals and alloys and cathodic reaction of oxidants in aqueous solutions. The corrosion rates depend on various factors such as materials, temperature, and solution chemistry. In addition they can change with duration time in the environments. Therefore, metallic corrosion are quite complex phenomena and it is quite difficult to predict life time of corroding metallic materials. In our laboratory, corrosion mechanisms are investigated mainly by advanced electrochemical techniques.
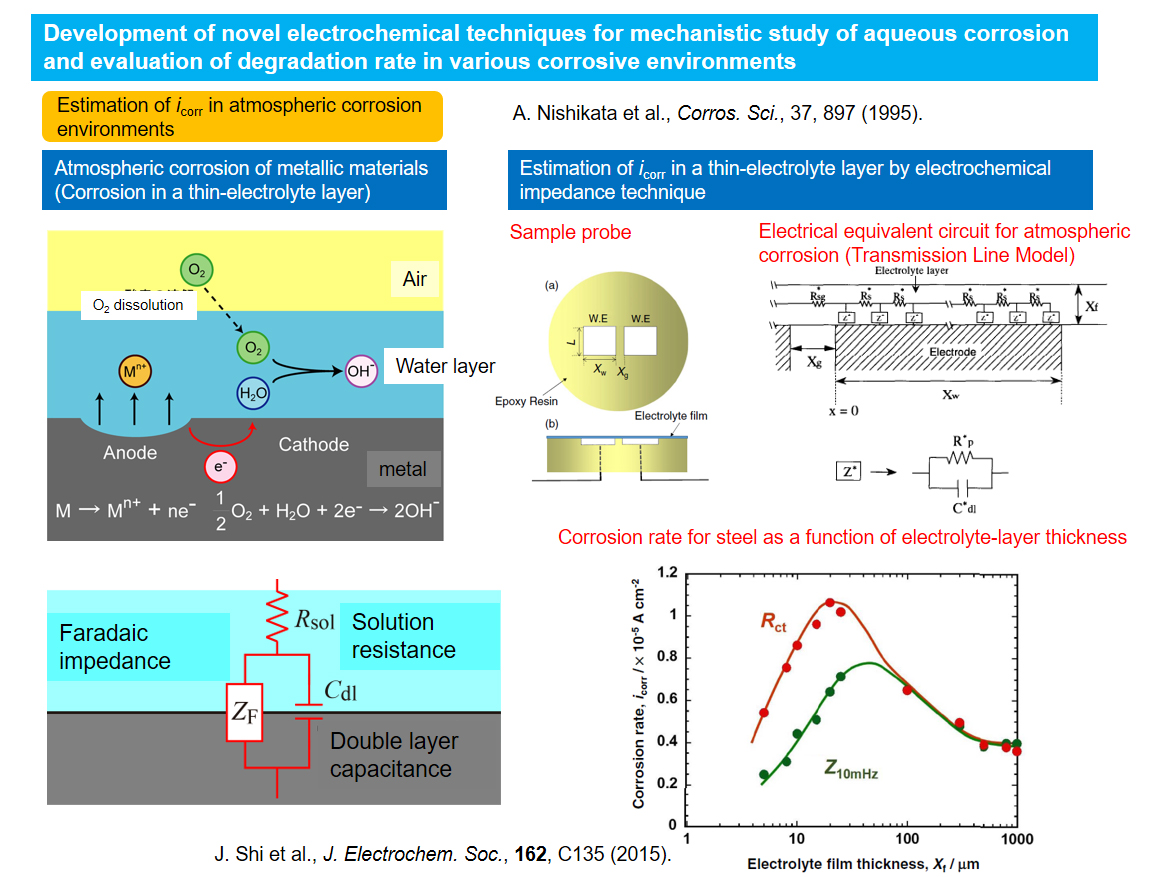
Development of novel electrochemical techniques for mechanistic study of aqueous corrosion and evaluation of degradation rate in various corrosive environments
Metallic corrosion proceeds at certain rates in various environments and causes changes of surface morphology by making dents and forming corrosion products. In order to evaluate the rates of corrosion degradation, we have to develop appropriate methods. In our laboratory, advanced, improved and unique electrochemical techniques such as electrochemical impedance spectroscopy (EIS) and micro-electrochemical proves are applied to evaluate of corrosion rates and to investigate corrosion mechanisms.
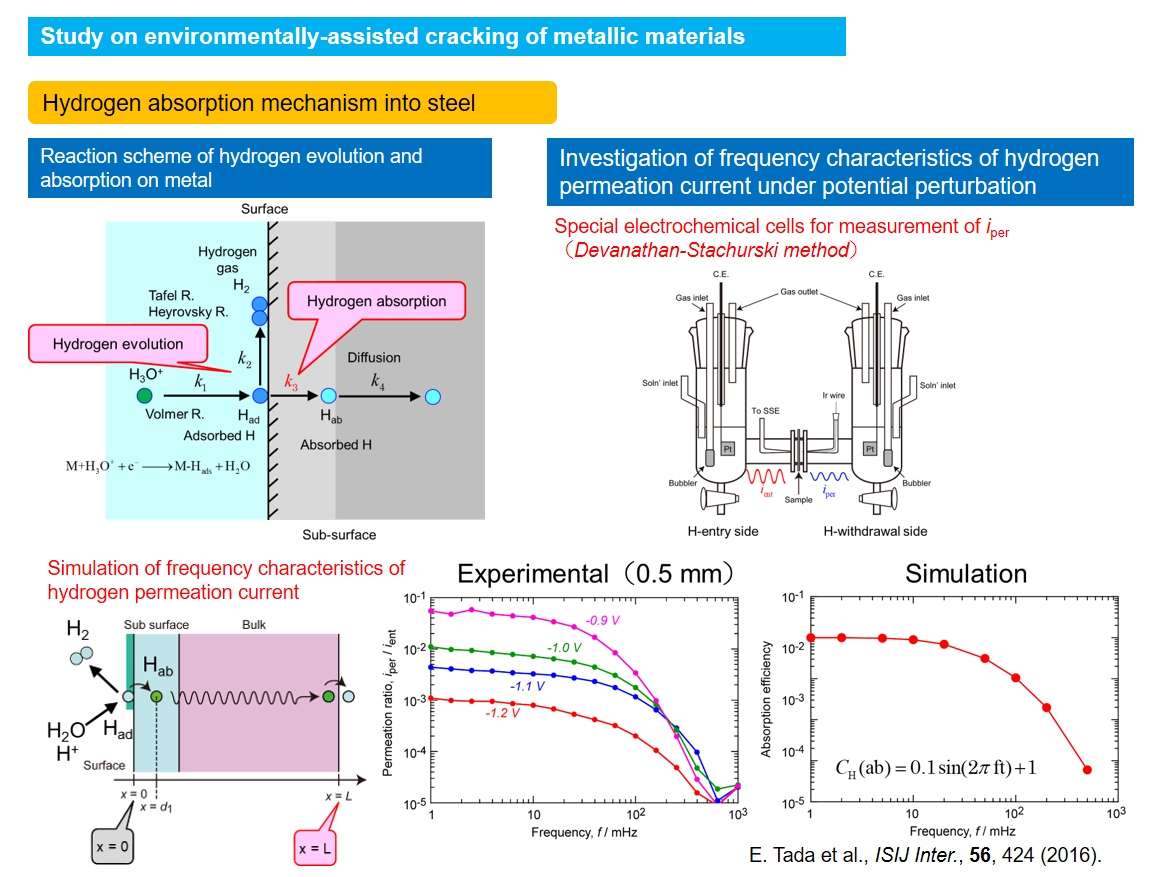
Study on environmentally-assisted cracking of metallic materials
Metallic materials have characteristics of relatively high strength and good ductility. However, cracking and fracture of metallic materials can be induced when they are used in corrosive environments. In our laboratory, hydrogen embrittlement (HE) which is the degradation on metallic materials induced by absorbed hydrogen and stress corrosion cracking of corrosion-resistant alloys (CRAs) such as stainless steels have been investigated by using various electrochemical methods.
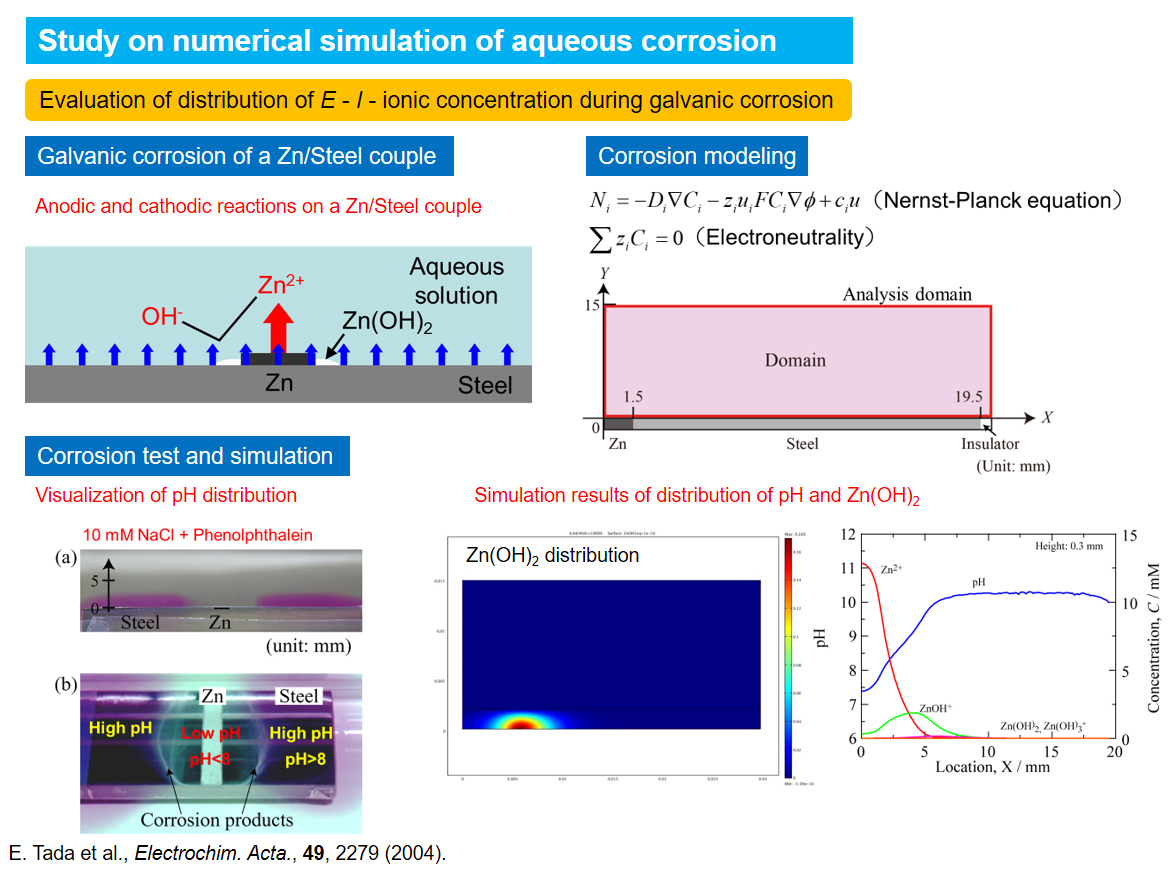
Study on numerical simulation of aqueous corrosion
In these days, processing speed of personal computers are improved faster and faster and we can utilize the computers to large-scale chemical systems with relatively low cost and relative ease. Although aqueous corrosion is one of complicated electrochemical reactions, computer simulation is expected to predict corrosion rates and elucidate degradation mechanisms. We are applying approaches by numerical simulation to corrosion fields to evaluate corrosion rates and investigate corrosion mechanisms.